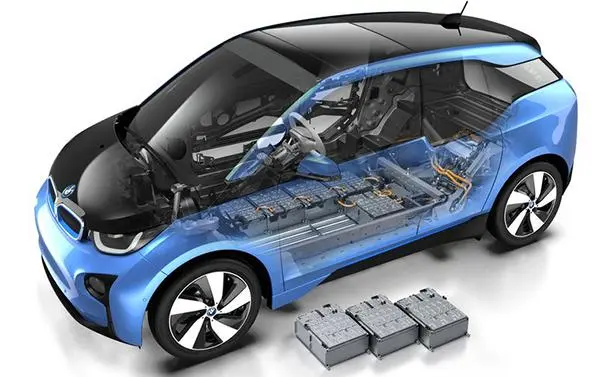
Electric vehicles are regularly in the news these days, largely because air quality has become a hot topic. Traditional fuel vehicles are one of the main sources of pollution, so governments around the world are trying to vigorously promote electric vehicles.
As an energy source for electric vehicles, battery technology has always been one of the key factors affecting the popularity of electric vehicles, and Lithiumbatterien are currently considered to be one of the most promising next-generation batteries. Let’s take a look at the types of Lithiumbatterien currently on the mainstream market:
The ternary lithium battery has a high energy density and short charging time. Its cycle charge and discharge is about 1000 times, and the theoretical life is 4 years.
Der lithium iron phosphate battery has a longer service life, the number of cycles of charge and discharge is about 2000 times, and the theoretical life is about 5 years.
The growing demand for electric vehicles has brought about the rapid development of Batterie technology. How to improve the life of Lithiumbatterien has become a concern and problem to be solved by automobile manufacturers and scientists in various countries.
As global electric vehicle sales have doubled, prices of battery materials such as lithium, nickel, manganese, and cobalt have soared, and supply chains for these raw materials have been rendered unsustainable by the pandemic. In order to make the battery not only perform better than the current electric vehicle battery but also meet the low cost of making materials, this has led scientists to turn their attention to the ubiquitous and cheaper sulfur. The natural abundance and chemical structure of sulfur make it Can store more energy.
A team of researchers at Drexel University recently published a study in the journal Communications Chemistry: They have developed a carbon nanofiber cathode to stably introduce sulfur into Lithium-Ionen-Batterien—and the results are astounding!
Carbonate electrolytes are the energy transfer liquids used in commercial lithium-ion batteries, and the challenge for Lithiumbatterien incorporating sulfur into carbonate electrolytes is the irreversible interaction of intermediate sulfur products, known as polysulfides, with the carbonate electrolyte. Due to this undesirable reaction, previous attempts to use sulfur cathodes in batteries with carbonate electrolyte solutions have resulted in near-impossible and complete Batterie failure after only one cycle.
To eliminate polysulfide formation to avoid adverse reactions, the team developed a carbon nanofiber cathode that attempts to use vapor deposition techniques to confine sulfur in the carbon nanofiber cathode substrate, slowing ether by reducing the movement of intermediate polysulfides Shuttle effect in Lithium-sulfur-based Batterien.
After more than a year of testing, the battery’s performance did not degrade over 4,000 charge-discharge cycles, which is equivalent to 10 years of normal use. And, as predicted, the battery has more than three times the capacity of a Lithium-Ionen-Akku.
“When we started testing, it started to work beautifully — something we didn’t expect. We suspected that the sulfur cathode would cause the reaction to stop, but it actually performed very well and didn’t cause shuttling.” Chief Kalra said.
This research has greatly improved the service life of electric vehicle batteries. The battery used to be replaced every three or four years, but now it can be replaced every ten years, which greatly reduces the cost of using electric vehicles! The Batterien of the future will be cheaper and cheaper.